We have previously developed and applied an in vivo model of sub-rupture fatigue damage accumulation using the rat and mouse patellar tendons. 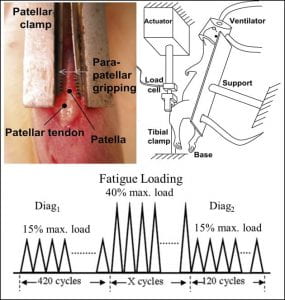 We have shown that sub-rupture damage can accumulate from just one bout of fatigue loading. We have found that tendons ineffectively repair accumulated sub-rupture fatigue damage and therefore become increasingly predisposed to further injury. This induced sub-rupture damage does not innately remodel, and early onset of damage subjects the tendon to a sustained risk of accumulation of further injury. Higher levels of severity of the initial damage induced prompt an increasingly muted and ineffective biological response, which ultimately results in diminished repair.
We have shown that sub-rupture damage can accumulate from just one bout of fatigue loading. We have found that tendons ineffectively repair accumulated sub-rupture fatigue damage and therefore become increasingly predisposed to further injury. This induced sub-rupture damage does not innately remodel, and early onset of damage subjects the tendon to a sustained risk of accumulation of further injury. Higher levels of severity of the initial damage induced prompt an increasingly muted and ineffective biological response, which ultimately results in diminished repair.
Despite this ineffective capacity of tendons to innately repair, our studies suggest that the biomechanical environment of the damaged dictates its capacity to respond to therapeutics. Ongoing studies are evaluating the mechanistic role of some of the mechanistic changes that occur after injury. Our goal is to determine whether some of the observed changes are manifestations of the tendon damage, or part of an attempt (albeit ineffective without further perturbation) to repair.
Ongoing projects:
- The mechanistic role of increased glycosaminoglycans in post-fatigue damaged tendons. We have found that the increase in GAGs, particularly dermatan sulfate (DS) and hyaluronan (HA), that is associated with late stage tendinopathy initiates after just 1 bout of fatigue loading. We are evaluating whether the early increase in DS and HA modulates the mechanical environment of the resident cells to restore interactions with the extracellular matrix (ECM) and promote survival; a mechanistic necessity for the fatigue damaged tendon to repair from subsequent therapeutic loading.
- The role of inflammation in tendinopathy. The role of inflammation in chronic tendinopathy has been a topic of great controversy. While inflammatory cells have been observed in clinical discards, it is unclear whether inflammation plays any role in the pathogenesis or the attempted (albeit ineffective) repair response of tendinopathy. We are investigating the potential role and therapeutic potential of modulating inflammation in tendinopathy.
Relevant publications:
- Bell R,* Robles-Harris M*, Anderson M, Laudier D, Schaffler M, Flatow EL, Andarawis-Puri N. Inhibition of apoptosis exacerbates fatigue damage tendon injuries in an in vivo rat model. Eur Cell Mater. 2018 Jul 30;36:44-56. *Contributed equally to this work.
- Andarawis-Puri N, Philip A, Laudier D, Schaffler MB, Flatow EL. Temporal effect of in vivo tendon fatigue loading on the apoptotic response explained in the context of number of fatigue loading cycles and initial damage parameters. J Orthop Res. 2014;32(9):1097-1103.
- Andarawis-Puri N, Sereysky JB, Sun HB, Jepsen KJ, Flatow EL. Molecular response of the patellar tendon to fatigue loading explained in the context of the initial induced damage and number of fatigue loading cycles. J Orthop Res. 2012;30(8):1327-1334.
- Sereysky JB, Andarawis-Puri N, Jepsen KJ, Flatow EL. Structural and mechanical effects of in vivo fatigue damage induction on murine tendon. J Orthop Res. 2012;30(6):965-972.
- Neviaser A, Andarawis-Puri N, Flatow E. Basic mechanisms of tendon fatigue damage. J Shoulder Elb Surg. 2012;21(2):158-163.
- Andarawis-Puri N, Sereysky JB, Jepsen KJ, Flatow EL. The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon. J Biomech. 2012;45(1):59-65.
- Andarawis-Puri N, Flatow EL. Tendon fatigue in response to mechanical loading. J Musculoskelet Neuronal Interact. 2011;11(2):106-114.
- Sun HB, Andarawis-Puri N, Li Y, et al. Cycle-dependent matrix remodeling gene expression response in fatigue-loaded rat patellar tendons. J Orthop Res. 2010;28(10):1380-1386.